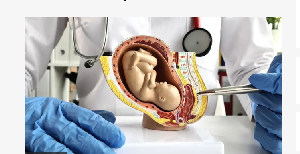
Artificial placentas and wombs fit save di lives of premature babies, but wetin dem suppose consider ethically bifor human trials go start?
E sound like plot wey come out straight from bad science fiction movie – human babies wey dem collect from dia mama belle and grow dem inside fluid-filled pods instead.
Yet na di exact tin wey scientists for di Children Hospital of Philadelphia (CHOP) for Pennsylvania for di US propose to do for infants wey dey at risk of extreme premature birth.
Dem dey develop wetin dem refer to as an "artificial womb", or extra-uterine environment for newborn development, wey dem dey also call (Extend).
Extend no dey intended to grow foetus from conception to birth – sake of say e go dey impossible even if e dey desirable.
Instead, di plan na to help boost di survival rate of extremely premature infants, wey dey face plenti health effects throughout dia lives.
Typical healthy pregnancy dey last around 40 weeks, and dem dey consider babies wey don mature to full term at 37 weeks.
However somtimes complications dey happun for pregnancy wey fit make di mother born di pikin early.
Luckily, sake of di huge advances for neonatal medicine ova di last few decades, most premature infants dey survive and dem dey discharge dem wit few complications.
Di most recent data show say even 30% of 22-week-gestation patients survive if dem give dem intensive care.
"Honestly, 28 weekers and even 27 weekers dey do veri well overall," na wetin Stephanie Kukora, one neonatologist for Children's Mercy Hospital for Kansas City tok.
"Na really di babies wey dem born at 22 to 23 weeks wey di outcomes dey so severe to di point wey we no dey sure weda di quality of life dem go get fit dey acceptable."
Babies wey dem born during dia transition from one stage to anoda dey face severe health challenges.
Dis infants weigh less dan 2lb (900g) wen dem born dem, and critical organs like di heart, di lungs, di digestive organs, and di brain neva develope well enough to keep di baby alive witout intensive medical care.
Short-term complications wey dey frequently arise include necrotising enterocolitis (NEC), dis na serious illness wen di tissues for di intestine (gut) become inflamed and start to die.
Infants of dis age dey also catch infection easily, like sepsis and septic shock – wey dey life threatening.
Na drop in blood pressure wey fit damage di lungs, kidneys, liver and oda organs.
Meanwhile long-term issues wey fit affect extremely premature babies include cerebral palsy, moderate to severe learning difficulties, vision and hearing problems, and asthma.
Even di very technology wey dem design to save di life of di babies' – oxygen support and ventilation – fit harm di lungs of dis infants' wey neva mature.
"For dat early gestational age di lungs still dey develop and fluid suppose full inside," George Mychaliska, wey be professor of surgery and obstetrics and gynaecology at Michigan University's C S Mott Children's Hospital tok.
"But wen dem born pikin very prematurely, we dey put endotracheal tube for dia trachea, and we force air and oxygen at high tension and pressure into dia lungs – wey dey well documented to cause injury."
Over time di injuries lead to scarring of di lungs, one condition wey dem know as bronchopulmonary dysplasia, or chronic lung disease.
Children dey leave hospital evritime and dem go still need long-term oxygen support and require mechanical ventilation for di rest of dia lives.
Ventilation fit also raise di risk of retinal blindness. Di blood vessels wey dey feed di eye’s retina no dey fully normal until close to birth.
Too much oxygen fit trigger di growth of new, abnormal blood vessels, wey fit ultimately lead to retinal detachment.
Di idea behind artificial wombs and placentas na to take di lungs out of di equation all togeda, to give di foetus time to kontinue to develop for a safe environment until di baby don dey ready to take im first breath.
How dis new technology go work?
E get three main groups wey dey work on di technology. All three take dia inspiration from an existing therapy called extracorporeal membrane oxygenation (Ecmo), na one type of artificial life support wey fit help pesin wey im lungs and heart no dey function properly.
In Ecmo, dem go pump blood outside of di patient bodi to one machine wey go remove carbon dioxide and add oxygen. Dem go send di oxygenated blood back to di tissues for di bodi.
Dis method go allow blood to "bypass" di heart and lungs, wey go allow dis organs to rest and heal.
Although dem fit use Ecmo on older babies, e no dey suitable for extremely premature infants.
All di three teams dey try to miniaturise and adapt di technology.
However, small small differences dey between di different devices in development.
Scientists for CHOP, wey one foetal surgeon Alan Flake, dey lead, plan to submerge premature babies inside fluid-filled pods designed to copy di amniotic fluid of di womb.
Surgeons go den connect di tiny blood vessels of di baby umbilical cord to one Ecmo-like device.
Dem go pump blood around di system using di foetal heart, just di way e dey happun naturally.
For 2017, Flake and im colleagues take eight premature lambs of an equivalent gestational age to 23-to-24-week-old human foetuses, dem keep dem alive for four weeks using di artificial womb.
During dis time dem see say di lambs dey develop normally, dem dey even grow wool.
George Mychaliska's team for di University of Michigan, on di oda hand, dey develop wetin dem call artificial placenta.
Instead of submerging di whole foetus inside fluid, dem dey plan to use breathing tubes to fill di lungs of di infant wit a specially developed fluid.
Dia system dey drain blood from di heart thru di jugular vein, similar to traditional Ecmo machines, but e go returns oxygenated blood thru di umbilical vein.
"I bin want a platform wey dey readily available to most babies, and e fit dey used for existing neonatal intensive-care units," Mychaliska tok.
"Di technology no dey intended to replace di many functions of di placenta. E dey focus on gas exchange and maintaining blood pressure, heart rate and foetal circulation while di premature organs dey protected and kontinu to develop."
For one recent trial of di artificial placenta, premature lambs wey dem keep for di machine survive for 16 days bifor dem transfer dem safely to mechanical ventilation.
During dis time dia lungs, brains and oda organs kontinu to develop well.
Di third group, one team from Australia and Japan, dey develop one artificial womb called ex vivo uterine environment (Eve) therapy.
Di aim na to treat more premature and sick foetuses dan di oda two groups.
"We dey for a point now wia we fit take 500g [lamb] foetus and maintain am for wetin I fit describe as a broadly normal physiological state for two weeks at a time," Matt Kemp, professor of obstetrics and gynaecology for di National University of Singapore, wey dey lead Eve tok.
"Na veri good neat achievement, but on di flip side, di growth of dis foetuses dey abnormal."
Most of di trials wey we bin conduct using artificial placentas/wombs wey dey on lamb foetuses wey dey healthy and e for go thru to term if dem no disturb am.
Di problem be say dem dey born extremely premature babies sake of health complications wey dey come from either di mother or di foetus itself. Diafore dem dey veri difficult to treat.
"For one experiment wey we don do with quite compromised foetuses, dos animals dey much more difficult to manage," Kemp tok.
"Dia growth dey far worse, and dia blood pressures and flow dey much, much more difficult to keep normal."
How soon we see artificial placentas and wombs for hospitals?
E be like say CHOP don go far well-well for di development pipeline. Di team bin recently apply to di Federal Drug Administration (FDA) for permission to begin human trials of Extend.
Mychaliska, on di oda hand, dey hopes to move to human clinical trials around three or four years, afta im team don reduce dia system further to cope wit di tiny blood vessels of human neonate.
However, Kemp still think say fundamental gaps still dey for for di knowledge of how foetuses grow for artificial wombs wey need filling in bifor we move to trials.
"We tink say e dey clear well-well say a very small foetus no need di ability to direct im own growth for a normal fashion, and e go dey worse wen e dey sick," Kemp tok.
"So we dey trying to unpack di involvement of di placenta to drive di normal growth processes. Dis na wia we don reach now, and na pretty big task, to put it mildly."
E get ethical considerations too. For one recent article, Stephanie Kukora argue say e get small differences between di distinct technologies wey create unique ethical challenges.
For example, as di artificial wombs of EVE and CHOP teams need fitting a cannula to di umbilical cord, dem need to transfer di babies immediately from di mother to di machine.
<>Complications or risks dey for dis procedure?
Sake of say di umbilical artery dey close quickly afta birth. Mothers wey suppose to born dia pikin naturally thru di vagina go den need to get early Caesarean section [CS].
"Wen you do Caesarean section veri early, dem no go fit do am di way wey dem dey normally do am for babies wey mature inside di womb." Kukora tok.
"E involve an incision a cut wey go pass thru di muscular layer of di uterus, and wey fit get an impact on future pregnancies, such as weda dem fit carry full pregnancy and deliver dia babies from di vagina again."
Dis procedure get plenti risks if dem compare am to di vaginal birth procedure wey raise issues to do wit informed consent.
"I think say one of di biggest ones na how we go approach expectant parents wen we wan do dis trial," Kukora tok.
"You fit imagine a parent wey dey facing dis really sad situation, wey just come out from counselling about di poor outcomes at 22 weeks, and wey fit dey really excited for something new even if demneva test am. Parents will do anytin for dia infant."
Anoda issue wit immediately transferring a baby onto di Extend system na say no opportunity to assess how di baby for make developmental progress for di on conventional therapy.
"You no get a lot of data apart from di gestational age to decide who go fit enta di Extend system – sake of say dem neva born di pikin yet, so you no know di babies dey do inside," Mychaliska tok.
Dis fit mean say babies wey bin suppose to do well on traditional therapies fit dey treated on a new untested technology, wey dem neva know di risks well-well.
However, Mychaliska believe say Extend go dey beneficial for di most premature infants wey dey 22-23 weeks gestational age, who dey known to suffer high mortality and morbidity.
As e dey drain blood from di jugular vein instead of di umbilical artery, doctors get more time to place babies on Mychaliska artificial placenta.
Dis go allow physicians to "risk stratify" babies afta birth, wit di aim say only di infants wey dey sick well-well na im dem go transfer to di treatment arm of di trial.
Dem fit also potentially treat Infants using conventional therapy first, bifor dem transfer dem to di artificial placenta at a later date if dem no dey do well.
Unlike di oda two technologies, mothers fir also deliver dia babies vaginally.
Any of di technology wey reach trials first, di first participants for di trials dey likely to be babies wey dem born bifor 24 weeks wey get veri poor chance of survival wit a good outcome using conventional treatment.
"I tink di technology go revolutionise di field of prematurity, and di artificial placenta and Extend approaches go dey complimentary for clinical practice," Mychaliska tok.
"But e get e own potential risks wey we need to assess for di initial trial of safety.
I tink di initial application of dis technology na for babies wey get a poor chance of survival, and den we fit expand am to more premature infants once we determine di risks and if di technology work well".
If e dey successful, all three technologies go offer a much-needed lifeline of hope to parents wey unexpectedly go into premature labour.

Africa News of Sunday, 28 July 2024
Source: bbc.com